A Z X Notation Chemistry
metako
Sep 10, 2025 · 8 min read
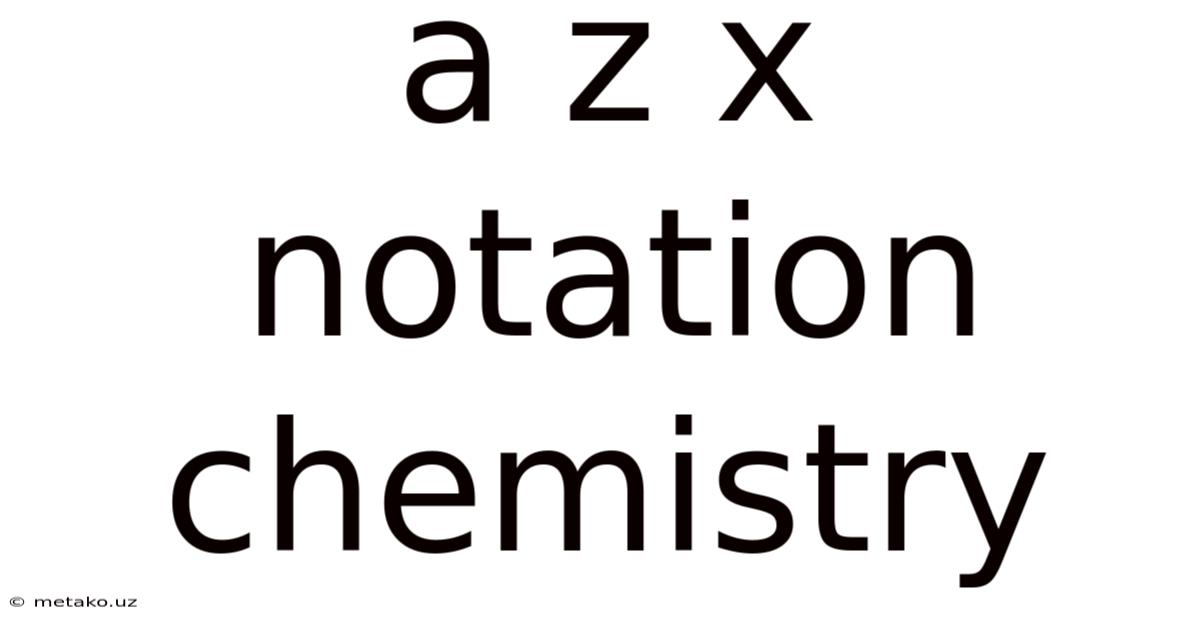
Table of Contents
Decoding the Mystery: A Comprehensive Guide to AXE Notation in Chemistry
Understanding molecular geometry is crucial in chemistry. It dictates a molecule's properties, reactivity, and ultimately, its role in the wider world. One of the most efficient ways to visualize and predict molecular geometry is through AXE notation, a simple yet powerful system that allows us to quickly grasp the arrangement of atoms and lone pairs around a central atom. This article will provide a thorough exploration of AXE notation, explaining its principles, applications, and limitations. We'll delve into examples, address frequently asked questions, and equip you with the knowledge to confidently apply this essential tool in your chemistry studies.
Introduction to AXE Notation: A Simple Yet Powerful Tool
AXE notation, also known as the Gillespie-Nyholm notation, is a shorthand method used to describe the arrangement of atoms and electron pairs around a central atom in a molecule. It’s a cornerstone of the Valence Shell Electron Pair Repulsion (VSEPR) theory, which posits that electron pairs, both bonding and non-bonding (lone pairs), repel each other and arrange themselves to minimize this repulsion, thereby determining the molecule's shape.
The notation itself is straightforward:
- A: Represents the central atom.
- X: Represents the number of atoms bonded to the central atom.
- E: Represents the number of lone pairs of electrons on the central atom.
For example, the AXE notation for water (H₂O) is AX₂E₂. This tells us that there is one central oxygen atom (A), two hydrogen atoms bonded to it (X₂), and two lone pairs of electrons on the oxygen atom (E₂). This notation allows us to quickly predict the molecular geometry (bent) based on the arrangement of atoms and lone pairs.
Understanding the Components of AXE Notation
Let's break down each component of the notation in more detail:
-
The Central Atom (A): This is typically the least electronegative atom in the molecule, although there can be exceptions. It's the atom around which the other atoms are arranged. In many cases, this is the atom with the highest number of bonds.
-
The Number of Bonding Atoms (X): This refers to the number of atoms directly bonded to the central atom. These bonds are formed by sharing electrons between the central atom and the surrounding atoms. The number of X's directly influences the overall molecular geometry.
-
The Number of Lone Pairs (E): These are pairs of valence electrons on the central atom that are not involved in bonding. They occupy space and significantly influence the molecular geometry, even though they don't directly connect to other atoms. Lone pairs exert a stronger repulsive force than bonding pairs.
Applying AXE Notation: A Step-by-Step Guide
To successfully use AXE notation, follow these steps:
-
Draw the Lewis Structure: Begin by drawing the Lewis structure of the molecule. This will show you the arrangement of atoms and valence electrons. Remember to follow the octet rule (or expanded octet rule for elements in Period 3 and beyond) when drawing the structure.
-
Identify the Central Atom: Determine the central atom. Usually, it's the least electronegative atom, often located in the center of the molecule.
-
Count the Bonding Atoms (X): Count the number of atoms directly bonded to the central atom.
-
Count the Lone Pairs (E): Count the number of lone pairs of electrons on the central atom. These are pairs of electrons that are not involved in bonding.
-
Write the AXE Notation: Combine the information from steps 3 and 4 to write the AXE notation. For example, if you have a central atom with three bonded atoms and one lone pair, the notation would be AX₃E.
Predicting Molecular Geometry Using AXE Notation
The AXE notation is directly linked to the predicted molecular geometry. Different AXE notations correspond to different three-dimensional arrangements of atoms. Here's a table summarizing some common AXE notations and their corresponding molecular geometries:
| AXE Notation | Electron Pair Geometry | Molecular Geometry | Example |
|---|---|---|---|
| AX₂ | Linear | Linear | BeCl₂ |
| AX₃ | Trigonal Planar | Trigonal Planar | BF₃ |
| AX₂E | Trigonal Planar | Bent | SO₂ |
| AX₄ | Tetrahedral | Tetrahedral | CH₄ |
| AX₃E | Tetrahedral | Trigonal Pyramidal | NH₃ |
| AX₂E₂ | Tetrahedral | Bent | H₂O |
| AX₅ | Trigonal Bipyramidal | Trigonal Bipyramidal | PCl₅ |
| AX₄E | Trigonal Bipyramidal | See-Saw | SF₄ |
| AX₃E₂ | Trigonal Bipyramidal | T-shaped | ClF₃ |
| AX₂E₃ | Trigonal Bipyramidal | Linear | XeF₂ |
| AX₆ | Octahedral | Octahedral | SF₆ |
| AX₅E | Octahedral | Square Pyramidal | BrF₅ |
| AX₄E₂ | Octahedral | Square Planar | XeF₄ |
Note: The electron pair geometry describes the arrangement of all electron pairs (bonding and lone pairs), while the molecular geometry describes the arrangement of only the atoms. Lone pairs influence the molecular geometry by repelling bonding pairs.
Beyond the Basics: Exceptions and Complex Cases
While AXE notation provides a powerful framework for understanding molecular geometry, it's important to acknowledge its limitations. Some molecules don't neatly fit into the simple AXE scheme. For instance:
-
Multiple Central Atoms: Molecules with more than one central atom require a more complex approach, often involving analyzing each central atom individually.
-
Resonance Structures: Molecules with resonance structures might exhibit geometries that are an average of the contributing structures. The AXE notation might not accurately reflect the instantaneous geometry in these cases.
-
Steric Effects: Bulky substituents can cause deviations from the ideal geometries predicted by VSEPR theory. These steric effects are not directly accounted for in the basic AXE notation.
Advanced Applications of AXE Notation: Predicting Polarity and Properties
AXE notation is not just a tool for predicting shape; it's also crucial for understanding other molecular properties:
-
Molecular Polarity: The presence of lone pairs and the electronegativity differences between atoms significantly influence a molecule's polarity. AXE notation helps visualize the distribution of electron density, allowing us to predict whether a molecule will be polar or nonpolar. For instance, a symmetrical molecule like AX₄ (e.g., CH₄) is generally nonpolar, while a molecule like AX₃E (e.g., NH₃) is polar due to the uneven distribution of electron density caused by the lone pair.
-
Bond Angles: The angles between bonds are directly related to the molecular geometry. AXE notation provides a foundation for predicting bond angles, though actual bond angles can be slightly different from idealized values due to various factors, such as lone pair repulsion.
-
Spectroscopic Analysis: Understanding molecular geometry is essential for interpreting spectroscopic data like infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. AXE notation provides a framework for predicting the number and types of vibrational modes and chemical shifts, aiding in spectral analysis.
Frequently Asked Questions (FAQ)
Q: What is the difference between electron pair geometry and molecular geometry?
A: Electron pair geometry describes the arrangement of all electron pairs (bonding and lone pairs) around the central atom. Molecular geometry only describes the arrangement of the atoms themselves. Lone pairs influence the molecular geometry, causing deviations from the electron pair geometry.
Q: Can AXE notation predict bond lengths?
A: No, AXE notation primarily predicts the arrangement of atoms and doesn't directly predict bond lengths. Bond lengths are influenced by factors like atomic radii and bond order.
Q: What happens when an atom violates the octet rule?
A: Atoms in the third period and beyond can have an expanded octet, meaning they can have more than eight electrons in their valence shell. In these cases, the AXE notation still applies, but the electron pair geometry and molecular geometry might be more complex.
Q: How does AXE notation relate to hybridization?
A: AXE notation and hybridization are closely related. The AXE notation can help predict the hybridization of the central atom. For example, an AX₄ molecule will likely have sp³ hybridization.
Q: Are there any limitations to using AXE notation?
A: While very useful, AXE notation simplifies complex interactions. It doesn't account for factors like steric hindrance or the exact effects of differing electronegativities on bond angles and lengths. It also becomes less straightforward with molecules possessing multiple central atoms or those exhibiting significant resonance.
Conclusion: Mastering AXE Notation for Enhanced Chemical Understanding
AXE notation is an invaluable tool for students and professionals in chemistry. Its simplicity belies its power in predicting molecular geometry, understanding molecular polarity, and interpreting spectroscopic data. By mastering this system, you significantly enhance your ability to visualize and understand the three-dimensional structure of molecules and their inherent properties. Remember to always start with the Lewis structure, carefully count the bonding atoms and lone pairs, and then use the AXE notation to predict the geometry. While it has limitations, AXE notation remains a fundamental concept in chemistry, providing a solid foundation for more advanced studies in molecular structure and reactivity. Through consistent practice and a deeper understanding of the principles behind VSEPR theory, you can confidently decode the mysteries of molecular geometry using this efficient and versatile notation system.
Latest Posts
Latest Posts
-
Milk Is Heterogeneous Or Homogeneous
Sep 10, 2025
-
Specific Heat At Constant Volume
Sep 10, 2025
-
Hydrogen Bonding In Acetic Acid
Sep 10, 2025
-
How To Draw Slope Fields
Sep 10, 2025
-
How Does Light Affect Transpiration
Sep 10, 2025
Related Post
Thank you for visiting our website which covers about A Z X Notation Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.