Delta G Greater Than 0
metako
Sep 13, 2025 · 7 min read
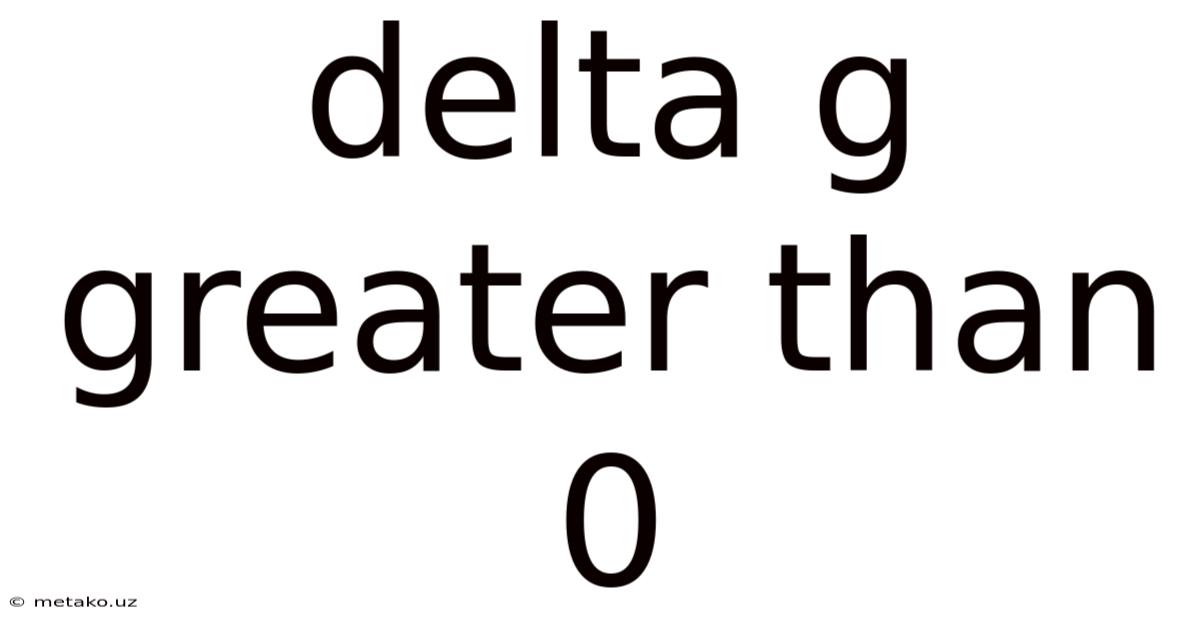
Table of Contents
Understanding Gibbs Free Energy and When ΔG > 0: A Deep Dive into Non-Spontaneous Processes
Understanding Gibbs Free Energy (ΔG) is crucial for comprehending the spontaneity of chemical and physical processes. While many focus on reactions where ΔG < 0 (spontaneous reactions), equally important is understanding situations where ΔG > 0, indicating a non-spontaneous process. This article delves into the meaning of a positive Gibbs Free Energy change, explores its implications, and provides illustrative examples to solidify your understanding. We'll also address common misconceptions and frequently asked questions to ensure a comprehensive grasp of this essential thermodynamic concept.
What is Gibbs Free Energy (ΔG)?
Gibbs Free Energy, denoted as ΔG, represents the maximum amount of reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. It's a state function, meaning its value depends only on the initial and final states of the system, not the path taken. The change in Gibbs Free Energy (ΔG) is calculated using the following equation:
ΔG = ΔH - TΔS
Where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy (heat content) of the system
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy (disorder) of the system
A negative ΔG indicates a spontaneous process (occurs without external intervention), while a positive ΔG signifies a non-spontaneous process (requires external energy input to proceed). A ΔG of zero represents a system at equilibrium.
Deciphering ΔG > 0: Non-Spontaneous Reactions
When ΔG is greater than zero (ΔG > 0), the reaction is non-spontaneous under the given conditions. This means that the reaction will not proceed naturally without an external driving force. The positive value reflects the fact that the increase in enthalpy (ΔH > 0, endothermic reaction) outweighs the increase in entropy (TΔS < ΔH) or a decrease in entropy (ΔS < 0) overwhelms the decrease in enthalpy (ΔH < 0).
Let's break this down:
-
Endothermic Reactions with Low Entropy Increase: Many endothermic reactions (those that absorb heat) are also characterized by a decrease in entropy (e.g., the formation of a more ordered crystalline structure). In these cases, both ΔH and TΔS contribute positively to ΔG, resulting in a significantly positive value, making the reaction highly non-spontaneous.
-
Exothermic Reactions with Significant Entropy Decrease: Even exothermic reactions (releasing heat) can be non-spontaneous if the decrease in entropy is substantial enough to outweigh the negative ΔH. For example, the freezing of water at temperatures above 0°C is an exothermic process, but the decrease in entropy (from liquid to solid) makes ΔG positive, hence it's non-spontaneous.
Factors Affecting the Spontaneity of a Reaction (ΔG)
Several factors influence whether ΔG will be positive or negative:
-
Temperature: Temperature plays a significant role, especially when the entropy change is substantial. An increase in temperature can make a non-spontaneous reaction spontaneous if the entropy increase is positive (TΔS term becomes larger).
-
Pressure: Changes in pressure primarily affect reactions involving gases. Increasing pressure can favor reactions that lead to a decrease in the number of gas molecules, potentially making a non-spontaneous reaction spontaneous.
-
Concentration: For reactions in solution, the concentrations of reactants and products affect the equilibrium constant and consequently the Gibbs Free Energy. Adjusting concentrations can shift the reaction towards spontaneity.
Illustrative Examples of ΔG > 0
Let's consider some practical examples to illustrate scenarios where ΔG > 0:
1. The Decomposition of Water:
The decomposition of water into hydrogen and oxygen gas is an endothermic reaction with a positive entropy change. However, at standard temperature and pressure, the positive enthalpy change is larger than the positive entropy contribution, leading to a positive ΔG. This explains why water doesn't spontaneously decompose into its constituent elements under normal conditions. External energy input, such as electrolysis, is required to drive this reaction.
2. The Formation of Ice from Water above 0°C:
As mentioned earlier, the freezing of water above 0°C is an example where a decrease in entropy outweighs the exothermic nature of the reaction. The positive ΔG indicates that ice will not spontaneously form from liquid water above its freezing point.
3. Protein Folding Under Certain Conditions:
While protein folding is generally a spontaneous process, certain conditions like high temperatures or the presence of denaturing agents can lead to an unfolded (denatured) state. In these cases, the process of folding the protein back into its native conformation might have a positive ΔG, requiring external energy or chaperone proteins to facilitate the process.
Coupling Non-Spontaneous Reactions with Spontaneous Reactions
One of the clever strategies employed in biological systems to overcome non-spontaneous reactions is coupling. A non-spontaneous reaction (ΔG > 0) can be driven forward by coupling it to a highly spontaneous reaction (ΔG << 0). The overall ΔG for the coupled reactions becomes negative, making the entire process spontaneous. This is a fundamental principle in biochemistry, particularly in metabolism where ATP hydrolysis provides the necessary driving force for many energy-requiring processes.
The Importance of Standard Gibbs Free Energy (ΔG°)
While ΔG considers actual conditions, the standard Gibbs free energy change (ΔG°) refers to the change in free energy under standard conditions (298 K and 1 atm pressure, and 1 M concentration for solutions). ΔG° is valuable for comparing the relative spontaneity of different reactions under consistent conditions. However, remember that ΔG° doesn't directly predict spontaneity under non-standard conditions. The actual ΔG under specific conditions needs to be calculated using the relationship:
ΔG = ΔG° + RTlnQ
Where:
- R is the gas constant
- T is the temperature in Kelvin
- Q is the reaction quotient
Frequently Asked Questions (FAQ)
Q1: Can a non-spontaneous reaction ever become spontaneous?
A1: Yes, by altering the reaction conditions such as temperature, pressure, or concentration, a reaction with a positive ΔG can become spontaneous (negative ΔG). As discussed earlier, this is often achieved by increasing the temperature for reactions with a positive entropy change or adjusting pressures in gas-phase reactions.
Q2: What is the difference between ΔG and ΔG°?
A2: ΔG represents the change in Gibbs Free Energy under actual reaction conditions, while ΔG° refers to the change under standard conditions (298 K, 1 atm, 1 M). ΔG° provides a useful benchmark, but ΔG is crucial for determining spontaneity under specific experimental circumstances.
Q3: How can I determine if a reaction is spontaneous without calculating ΔG?
A3: While calculating ΔG is the most accurate method, some qualitative assessments can be made. If the reaction is highly exothermic (releases a large amount of heat) and results in an increase in entropy (increased disorder), it's likely to be spontaneous. Conversely, endothermic reactions (absorbing heat) that lead to a significant decrease in entropy are generally non-spontaneous. However, these are only general guidelines, and ΔG calculation is always necessary for a definitive answer.
Q4: Is it possible to have a reaction with a ΔG of infinity?
A4: No, Gibbs Free Energy is a finite quantity. A reaction may be practically impossible to proceed under certain conditions (extremely positive ΔG), but the value itself remains finite.
Conclusion
Understanding Gibbs Free Energy and its implications, particularly when ΔG > 0, is fundamental to comprehending chemical and physical processes. While reactions with negative ΔG proceed spontaneously, those with positive ΔG require external energy input. However, manipulating reaction conditions or coupling with spontaneous reactions can shift the balance, making initially non-spontaneous processes feasible. The detailed explanation provided here, encompassing the underlying principles, examples, and frequently asked questions, should equip you with a solid understanding of this crucial thermodynamic concept. Remember that while standard Gibbs Free Energy offers a useful comparison point, determining spontaneity under specific conditions necessitates calculating the actual Gibbs Free Energy (ΔG) using the appropriate formula.
Latest Posts
Latest Posts
-
Normal Component Of Acceleration Formula
Sep 13, 2025
-
Non Example Of Sedimentary Rock
Sep 13, 2025
-
Diffraction Grating Vs Double Slits
Sep 13, 2025
-
Posterior View Of Skeletal System
Sep 13, 2025
-
Political Results Of Industrial Revolution
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Delta G Greater Than 0 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.