Electron Dot Structure Of Al
metako
Sep 19, 2025 · 6 min read
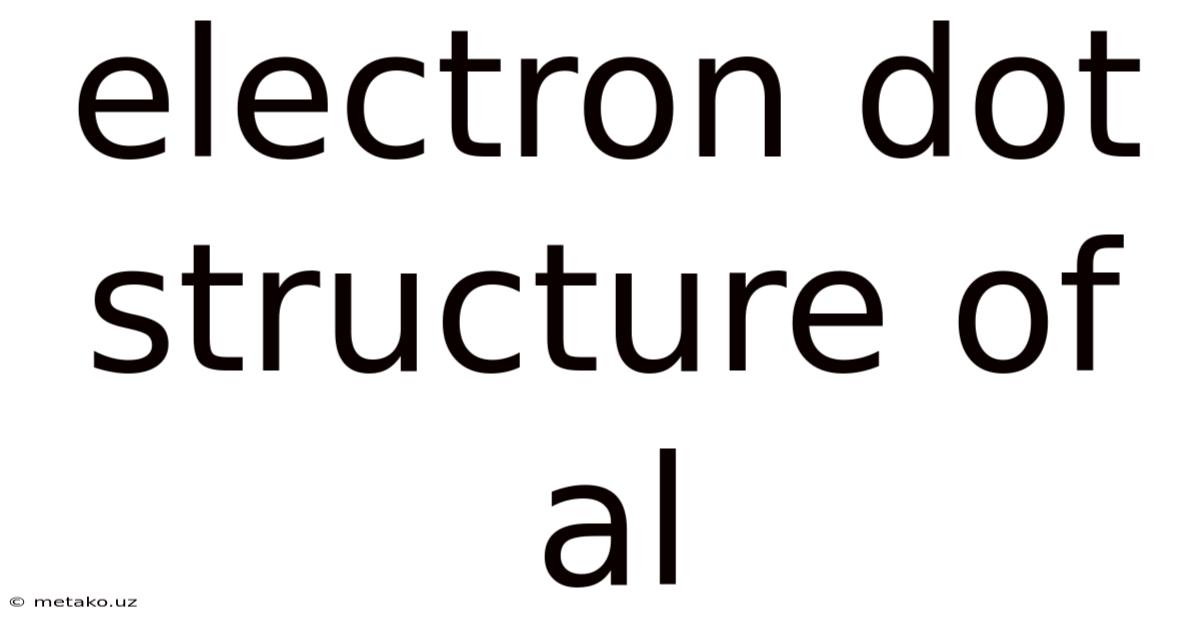
Table of Contents
Unveiling the Secrets of Aluminum's Electron Dot Structure: A Comprehensive Guide
Understanding the electron dot structure, also known as the Lewis dot structure, is fundamental to grasping the behavior of elements and how they form chemical bonds. This comprehensive guide will delve into the electron dot structure of aluminum (Al), exploring its creation, implications for bonding, and its significance in chemistry. We will also address common questions and misconceptions surrounding this vital concept. This article will equip you with a solid understanding of aluminum's electronic configuration and its role in various chemical reactions.
Introduction to Electron Dot Structures
Before focusing on aluminum specifically, let's establish a foundational understanding of electron dot structures. These diagrams visually represent the valence electrons – the electrons in the outermost shell of an atom – which are crucial in determining an atom's reactivity and bonding capabilities. Each dot surrounding the element's symbol represents a single valence electron. Understanding these structures provides a simplified yet powerful way to predict how atoms will interact to form molecules and compounds.
Determining Aluminum's Electron Configuration
To construct the electron dot structure of aluminum, we must first determine its electron configuration. Aluminum, with an atomic number of 13, has 13 electrons. These electrons fill atomic orbitals according to the Aufbau principle and Hund's rule. The electron configuration of aluminum is 1s²2s²2p⁶3s²3p¹. This translates to two electrons in the first shell (1s), eight electrons in the second shell (2s²2p⁶), and three electrons in the third shell (3s²3p¹).
Constructing the Aluminum Electron Dot Structure
The key to creating the electron dot structure lies in identifying the valence electrons. Remember, only the electrons in the outermost shell (the highest principal quantum number) are valence electrons. In aluminum's case, these are the three electrons in the third shell (3s²3p¹).
Therefore, the electron dot structure for aluminum is represented as:
•Al•
The symbol "Al" represents the aluminum atom, and the three dots surrounding it represent its three valence electrons. Note that these dots are usually placed individually around the element symbol before pairing up to visually represent the filling of orbitals, although this detail is less important at this introductory level.
Aluminum's Bonding Behavior: Implications of its Electron Dot Structure
The electron dot structure reveals a crucial aspect of aluminum's chemical behavior: its tendency to lose electrons to achieve a stable octet. Aluminum has three valence electrons, and losing these three electrons allows it to attain a stable electron configuration similar to that of neon (1s²2s²2p⁶), a noble gas with a filled outer shell. This drive to achieve a stable octet is the driving force behind aluminum's chemical reactivity.
Aluminum's Formation of Ionic Bonds
Aluminum readily forms ionic bonds with nonmetals, particularly halogens (Group 17 elements) and oxygen (Group 16). In these bonds, aluminum loses its three valence electrons to become a positively charged ion, Al³⁺, while the nonmetal gains these electrons to become a negatively charged ion. The electrostatic attraction between these oppositely charged ions forms the ionic bond.
For example, in aluminum chloride (AlCl₃), aluminum loses three electrons to three chlorine atoms, each of which gains one electron to achieve a stable octet. The resulting ions, Al³⁺ and Cl⁻, are held together by strong electrostatic forces.
Aluminum's Formation of Metallic Bonds
Aluminum is a metal, and its atoms are held together by metallic bonds within the solid aluminum structure. These bonds are distinct from ionic and covalent bonds. In metallic bonding, the valence electrons are delocalized and move freely throughout the metal lattice. This "sea" of delocalized electrons allows for the excellent conductivity of electricity and heat that characterizes metals like aluminum. The electron dot structure doesn't directly visualize metallic bonding, but it highlights the presence of valence electrons that participate in this type of bonding.
Comparing Aluminum's Bonding to Other Elements
It's instructive to compare aluminum's bonding behavior to that of other elements, especially those in the same group (Group 13). For instance, boron (B), with only three valence electrons, also tends to form covalent bonds but often exhibits a less complete octet in its compounds. Gallium (Ga), indium (In), and thallium (Tl), also in Group 13, display similar bonding behavior to aluminum, although subtle differences arise due to relativistic effects in the heavier elements. Understanding these similarities and differences helps establish a broader perspective on periodic trends.
Aluminum Compounds and Their Applications: A Practical Perspective
The unique bonding properties of aluminum, as reflected in its electron dot structure, lead to a wide array of important compounds and applications. Aluminum oxide (Al₂O₃), for instance, is a very hard, high-melting-point compound used in abrasives and ceramics. Aluminum sulfate (Al₂(SO₄)₃) is employed in water purification and as a mordant in dyeing. Organoaluminum compounds are utilized as catalysts in various industrial processes. The versatility of aluminum compounds stems directly from aluminum's ability to form stable ionic and covalent bonds, facilitated by its three valence electrons.
Advanced Concepts and Further Exploration
While the electron dot structure provides a simplified representation, it's important to acknowledge its limitations. It doesn't fully capture the complexities of bonding in transition metals or the nuances of molecular orbital theory. For a deeper understanding of aluminum's bonding behavior, exploring concepts like hybridization and molecular orbital theory becomes necessary. These advanced concepts build upon the fundamental understanding provided by the electron dot structure.
Frequently Asked Questions (FAQs)
Q: Can aluminum form covalent bonds?
A: While primarily forming ionic bonds, aluminum can participate in covalent bonding, particularly with less electronegative elements or in situations where electron deficiency is favored. However, these covalent bonds are typically less prominent than the ionic interactions.
Q: What is the difference between the electron dot structure and the electron configuration?
A: The electron configuration provides a complete description of how all electrons are distributed among the atomic orbitals, while the electron dot structure focuses solely on the valence electrons and their arrangement, providing a simplified visual representation relevant to bonding.
Q: Why is understanding the electron dot structure important?
A: Understanding the electron dot structure provides a simple but effective tool to predict the bonding behavior of elements, allowing for a qualitative understanding of chemical reactions and the formation of compounds.
Q: Are there exceptions to the octet rule in aluminum compounds?
A: Yes, while aluminum strives for a stable octet, there are exceptions, especially in situations with strong electronegative elements or steric constraints that influence bonding.
Conclusion: The Significance of Aluminum's Electron Dot Structure
In summary, the electron dot structure of aluminum, with its three valence electrons represented as three dots around the "Al" symbol, serves as a fundamental tool to understand its chemical behavior. This simple yet powerful representation directly relates to aluminum's propensity to lose three electrons to form stable ionic bonds or participate in metallic bonding within its metallic solid structure. The information provided by the electron dot structure forms the bedrock for understanding aluminum's reactivity and the properties of a vast array of important aluminum compounds, highlighting its crucial role in various scientific and technological applications. While more advanced theories offer a deeper understanding, mastering the basics of the electron dot structure is an essential stepping stone towards a comprehensive grasp of chemistry.
Latest Posts
Latest Posts
-
What Color Is A Ribosome
Sep 19, 2025
-
1 To 1 Function Examples
Sep 19, 2025
-
Genotypic Ratio For Dihybrid Cross
Sep 19, 2025
-
How To Find Percentage Mass
Sep 19, 2025
-
Measures Of Center And Spread
Sep 19, 2025
Related Post
Thank you for visiting our website which covers about Electron Dot Structure Of Al . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.