Enthalpy Change Of Solution Equation
metako
Sep 21, 2025 · 7 min read
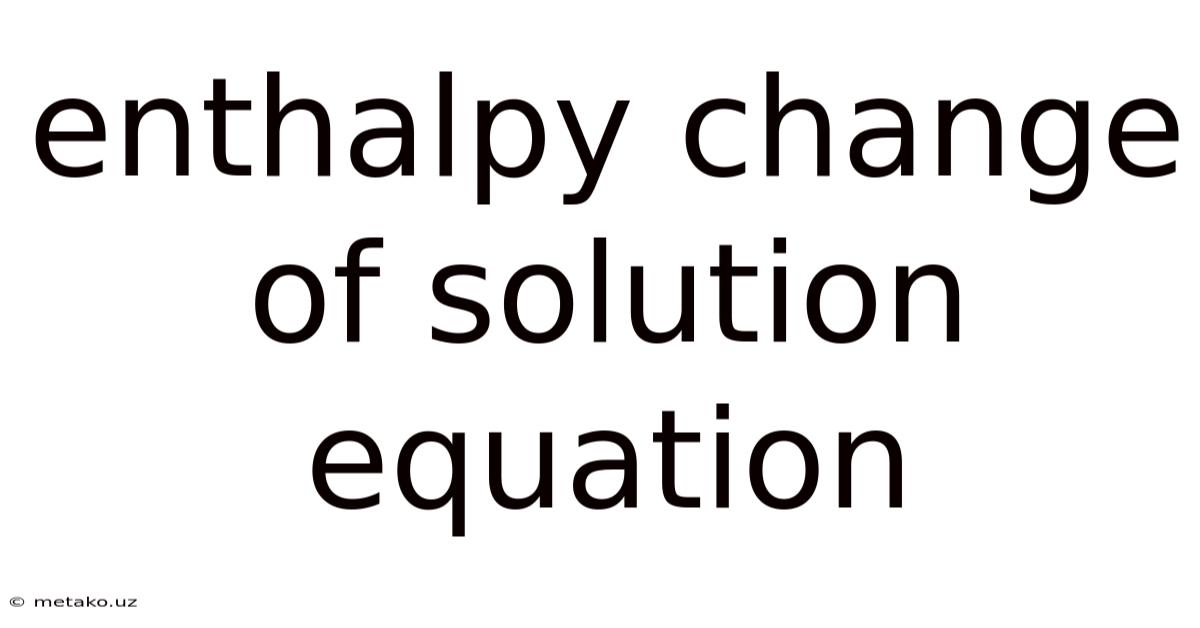
Table of Contents
Understanding the Enthalpy Change of Solution Equation: A Deep Dive
The enthalpy change of solution, often represented as ΔH<sub>sol</sub>, is a crucial concept in chemistry that describes the heat absorbed or released when a substance dissolves in a solvent. Understanding this process involves more than just memorizing an equation; it necessitates grasping the underlying principles of intermolecular forces, energy changes, and the factors influencing the overall enthalpy change. This comprehensive guide will delve into the enthalpy change of solution equation, exploring its components, applications, and practical implications.
Introduction: What is Enthalpy Change of Solution?
When a solute dissolves in a solvent, several interactions are broken and formed. These include solute-solute interactions (between particles of the solute), solvent-solvent interactions (between particles of the solvent), and solute-solvent interactions (between particles of the solute and solvent). The overall enthalpy change (ΔH<sub>sol</sub>) is the net result of these energetic changes. If more energy is released in forming new solute-solvent interactions than is absorbed in breaking solute-solute and solvent-solvent interactions, the process is exothermic (ΔH<sub>sol</sub> < 0), and heat is released into the surroundings. Conversely, if more energy is absorbed in breaking interactions than is released in forming new ones, the process is endothermic (ΔH<sub>sol</sub> > 0), and heat is absorbed from the surroundings. This heat exchange can be significant and affects many real-world processes, from making instant coffee to designing chemical reactions. This article will provide you with a thorough understanding of this fundamental concept.
The Enthalpy Change of Solution Equation: A Step-by-Step Breakdown
While there isn't a single, universally applicable equation to directly calculate ΔH<sub>sol</sub>, we can understand it through a series of related steps and enthalpy changes. The overall enthalpy change of solution is the sum of three key enthalpy changes:
-
Lattice Enthalpy (ΔH<sub>lattice</sub>): This is the enthalpy change associated with breaking the bonds in the solid solute to form gaseous ions. For ionic compounds, this involves separating the positive and negative ions, requiring significant energy input. This is always an endothermic process (ΔH<sub>lattice</sub> > 0).
-
Hydration Enthalpy (ΔH<sub>hydration</sub>): This is the enthalpy change when gaseous ions are surrounded by solvent molecules (hydration in the case of water). The ions attract the polar solvent molecules, forming ion-dipole interactions. This is generally an exothermic process (ΔH<sub>hydration</sub> < 0) because energy is released as these interactions are formed.
-
Enthalpy Change of Solution (ΔH<sub>sol</sub>): The overall enthalpy change of solution is the sum of the lattice enthalpy and the hydration enthalpy:
ΔH<sub>sol</sub> = ΔH<sub>hydration</sub> + ΔH<sub>lattice</sub>
This equation highlights the interplay between the energy required to break the solute's structure and the energy released when the solute interacts with the solvent. The magnitude and sign of ΔH<sub>sol</sub> depend on the relative magnitudes of ΔH<sub>hydration</sub> and ΔH<sub>lattice</sub>.
Factors Affecting Enthalpy Change of Solution
Several factors influence the magnitude and sign of ΔH<sub>sol</sub>:
-
Nature of the Solute: Ionic compounds generally have high lattice enthalpies due to strong electrostatic interactions. The charge density of the ions plays a crucial role; higher charge density leads to stronger interactions and larger lattice enthalpies. Molecular compounds, on the other hand, have lower lattice enthalpies because the intermolecular forces (such as van der Waals forces or hydrogen bonds) are weaker.
-
Nature of the Solvent: Polar solvents, like water, are effective at hydrating ions due to their ability to form strong ion-dipole interactions. Nonpolar solvents, however, have limited ability to interact with ions, leading to less exothermic hydration enthalpies. The dielectric constant of the solvent also plays a role; higher dielectric constants reduce the electrostatic interactions between ions, making it easier to dissolve the solute.
-
Temperature: Temperature affects the kinetic energy of the particles. At higher temperatures, particles have more kinetic energy, increasing the likelihood of successful collisions and facilitating the dissolution process. The temperature dependence of enthalpy itself is also a factor to consider.
-
Concentration: The enthalpy change of solution can vary slightly with concentration, particularly at higher concentrations where solute-solute interactions become more significant.
-
Pressure: The effect of pressure on the enthalpy change of solution is generally small for solids and liquids dissolving in liquids, but it can be more significant for gases dissolving in liquids.
Examples of Enthalpy Change of Solution
Let's illustrate with some examples:
-
Dissolving Sodium Chloride (NaCl) in Water: NaCl has a high lattice enthalpy. However, the hydration enthalpy of Na<sup>+</sup> and Cl<sup>-</sup> ions is even more significant and exothermic. This results in a slightly exothermic overall enthalpy change of solution for NaCl in water. The heat released is noticeable as a slight temperature increase.
-
Dissolving Ammonium Nitrate (NH<sub>4</sub>NO<sub>3</sub>) in Water: The dissolution of ammonium nitrate is endothermic. While hydration enthalpy is exothermic, the lattice enthalpy is relatively high, and the overall enthalpy change is positive. You can observe this as a decrease in temperature when ammonium nitrate dissolves in water.
Applications of Enthalpy Change of Solution
Understanding the enthalpy change of solution has many practical applications:
-
Chemical Engineering: Designing efficient chemical processes often requires understanding the heat generated or absorbed during dissolution. This is crucial for controlling reaction temperature and managing energy efficiency.
-
Pharmaceutical Industry: Dissolution is a critical step in drug delivery. Knowing the enthalpy change of solution helps in formulating drugs and predicting their solubility and bioavailability.
-
Environmental Science: Understanding how pollutants dissolve in water bodies is essential for assessing environmental impact and designing remediation strategies.
-
Material Science: Dissolution processes are important in material synthesis and processing, particularly in the creation of solutions used in various applications.
Frequently Asked Questions (FAQ)
Q: Can we directly measure ΔH<sub>sol</sub> experimentally?
A: Yes, ΔH<sub>sol</sub> can be measured experimentally using calorimetry. A calorimeter measures the heat absorbed or released during a process, allowing for the determination of the enthalpy change.
Q: Is the enthalpy change of solution always constant?
A: No, it can vary depending on the factors discussed earlier, such as temperature, concentration, and the nature of the solute and solvent.
Q: How does the enthalpy change of solution relate to solubility?
A: While an exothermic enthalpy change of solution (ΔH<sub>sol</sub> < 0) generally suggests higher solubility, it's not a definitive predictor. Entropy (disorder) also plays a critical role in determining solubility. Some compounds with endothermic enthalpy changes of solution are still quite soluble due to favorable entropy changes.
Q: What is the difference between enthalpy and entropy in the context of dissolution?
A: Enthalpy (ΔH) refers to the heat content of the system, while entropy (ΔS) represents the disorder or randomness. Both contribute to the Gibbs Free Energy (ΔG), which determines the spontaneity of a process. A negative ΔG indicates a spontaneous process (i.e., the substance will dissolve). While enthalpy change favors exothermic processes, entropy change often favors dissolution due to increased disorder.
Q: Are there any limitations to using the equation ΔH<sub>sol</sub> = ΔH<sub>hydration</sub> + ΔH<sub>lattice</sub>?
A: This equation provides a useful conceptual framework, but it's a simplification. It assumes the ions are completely separated in the gaseous state before hydration. In reality, the process is more complex, involving various intermediate steps and interactions.
Conclusion: The Significance of Enthalpy Change of Solution
The enthalpy change of solution is a fundamental concept with far-reaching implications across numerous scientific disciplines. Understanding the interplay between lattice enthalpy and hydration enthalpy provides a deeper understanding of dissolution processes. While the simplified equation offers a valuable introduction, the complete picture involves a more complex interplay of forces and factors. By appreciating these nuances, we can better predict and control dissolution processes in various applications, from chemical engineering to drug formulation and environmental science. The detailed insights provided here aim to equip you with a thorough understanding of this essential chemical concept.
Latest Posts
Latest Posts
-
Trailer Whats Eating Gilbert Grape
Sep 21, 2025
-
Serial Processing Vs Parallel Processing
Sep 21, 2025
-
Dna Digestion With Restriction Enzymes
Sep 21, 2025
-
Map Of North America Biomes
Sep 21, 2025
-
Body Covering Of A Reptile
Sep 21, 2025
Related Post
Thank you for visiting our website which covers about Enthalpy Change Of Solution Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.