Is H2o A Good Nucleophile
metako
Sep 06, 2025 · 6 min read
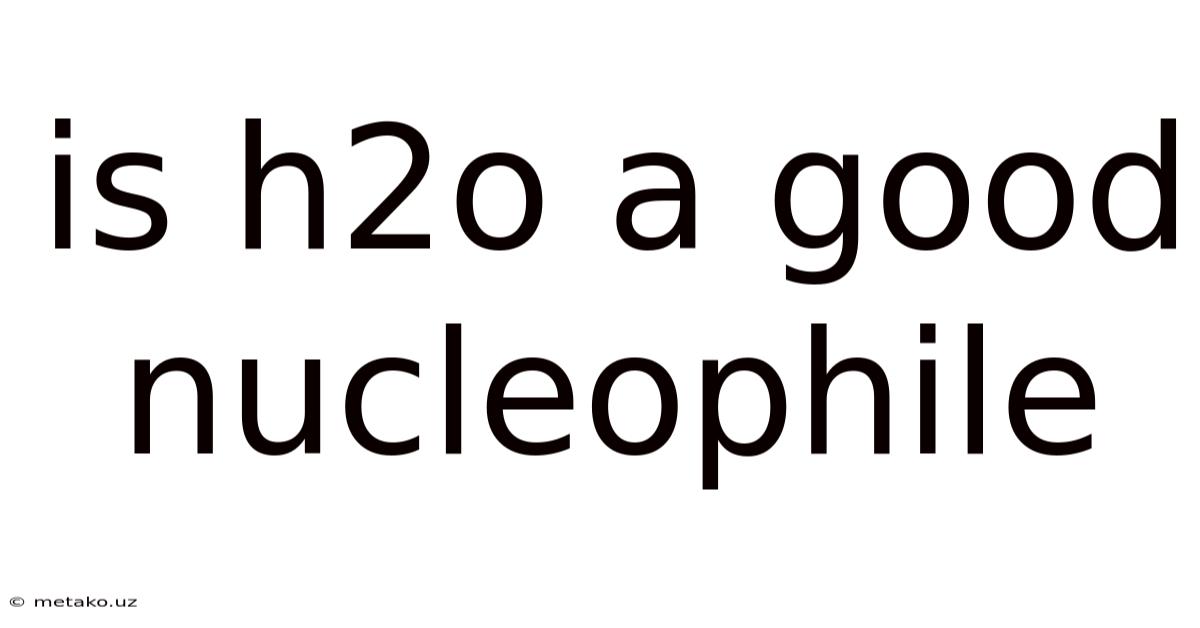
Table of Contents
Is H₂O a Good Nucleophile? A Deep Dive into Nucleophilicity
Water (H₂O), a ubiquitous molecule essential for life, often sparks discussion regarding its nucleophilicity. Is it a good nucleophile? The answer, as with many things in chemistry, is nuanced and depends heavily on the context. This article will delve into the factors influencing water's nucleophilic behavior, exploring its strengths and weaknesses as a nucleophile, comparing it to other common nucleophiles, and examining its role in various chemical reactions. Understanding water's nucleophilic character is crucial for comprehending a wide array of chemical processes, from biological reactions to organic synthesis.
Understanding Nucleophilicity
Before assessing water's nucleophilic prowess, let's define the term. A nucleophile (literally, "nucleus-loving") is a chemical species that donates an electron pair to an electrophile (an electron-deficient species) to form a chemical bond. The strength of a nucleophile, its nucleophilicity, is determined by several factors:
-
Charge: Negatively charged nucleophiles are generally stronger than neutral ones because the negative charge increases electron density, making them more readily available for donation.
-
Electronegativity: Less electronegative atoms are better nucleophiles because they hold their electrons less tightly, making them more easily shared.
-
Steric hindrance: Bulky nucleophiles can be hindered from approaching the electrophile, reducing their effectiveness.
-
Solvent effects: The solvent plays a crucial role. Protic solvents (those with an O-H or N-H bond) can solvate (surround) the nucleophile, reducing its reactivity. Aprotic solvents (lacking O-H or N-H bonds) generally enhance nucleophilicity.
Water as a Nucleophile: Strengths and Weaknesses
Water, with its lone pairs of electrons on the oxygen atom, can act as a nucleophile. However, its nucleophilicity is moderate compared to other, stronger nucleophiles.
Strengths:
-
Abundance and Accessibility: Water is readily available and inexpensive, making it a convenient nucleophile for many reactions.
-
Amphoteric Nature: Water's amphoteric nature allows it to act as both an acid and a base, contributing to its versatility in various chemical environments. This means it can participate in a wide range of reactions, even acting as a leaving group in certain cases.
-
Participation in Hydrolysis Reactions: Water excels as a nucleophile in hydrolysis reactions, where it attacks an electrophilic carbon atom, breaking a bond and incorporating the hydroxyl group (-OH). This is fundamental in many biological processes and organic chemistry reactions. Examples include the hydrolysis of esters, amides, and other functional groups.
Weaknesses:
-
Weak Nucleophile in Many Cases: Compared to stronger nucleophiles like hydroxide (OH⁻), alkoxides (RO⁻), or amines (R₃N), water's nucleophilicity is relatively weak. Its neutral charge and high electronegativity of oxygen limit its electron-donating ability.
-
Protic Solvent Effect: Water's protic nature hinders its nucleophilicity. The hydrogen bonding between water molecules significantly solvates the nucleophile (itself!), reducing its ability to approach and attack the electrophilic center.
-
Competitive Reactions: In many reactions, water can compete with other reactants, leading to lower yields or the formation of undesired products. This is especially true when dealing with reactive electrophiles or competing stronger nucleophiles.
Comparing H₂O to other Nucleophiles
Let's compare water's nucleophilicity to some common nucleophiles:
| Nucleophile | Charge | Electronegativity | Steric Hindrance | Nucleophilicity | Solvent Effect |
|---|---|---|---|---|---|
| H₂O | Neutral | High | Low | Weak | Reduced in protic solvents |
| OH⁻ | Negative | High | Low | Strong | Reduced in protic solvents, enhanced in aprotic solvents |
| CH₃O⁻ | Negative | High | Low | Strong | Reduced in protic solvents, enhanced in aprotic solvents |
| NH₃ | Neutral | Moderate | Low | Moderate | Reduced in protic solvents |
| I⁻ | Negative | Low | Low | Strong | Enhanced in aprotic solvents |
As we can see, hydroxide ion (OH⁻) is significantly stronger than water due to its negative charge. Alkoxides (RO⁻) also exhibit stronger nucleophilicity. Iodide (I⁻), despite its negative charge, is a powerful nucleophile, particularly in aprotic solvents. Ammonia (NH₃) shows moderate nucleophilicity, affected by solvent interactions.
The Role of H₂O in Specific Reactions
Water's role as a nucleophile varies considerably depending on the reaction conditions and the nature of the electrophile.
-
Hydrolysis of Esters: Water acts as a nucleophile, attacking the carbonyl carbon of an ester. This leads to the formation of a carboxylic acid and an alcohol. The reaction rate is often slow without a catalyst (acid or base).
-
Acid-Catalyzed Hydrolysis: In acid-catalyzed hydrolysis, the carbonyl group of the ester is protonated, making it a better electrophile, increasing the reaction rate with water.
-
Base-Catalyzed Hydrolysis: In base-catalyzed hydrolysis, the hydroxide ion (OH⁻) acts as a stronger nucleophile, attacking the ester, leading to faster hydrolysis than with water alone.
-
SN1 and SN2 Reactions: Water can participate in both SN1 (substitution nucleophilic unimolecular) and SN2 (substitution nucleophilic bimolecular) reactions. However, its effectiveness is limited compared to stronger nucleophiles. In SN1 reactions, its role is often secondary, participating in the subsequent step after the carbocation formation.
-
Biological Systems: Water's nucleophilic properties are crucial in biological systems. It participates in numerous enzymatic reactions, often acting as a reactant or assisting in the formation of reactive intermediates.
Frequently Asked Questions (FAQ)
Q: Can water ever be a good nucleophile?
A: While not as strong as many other nucleophiles, water can act as a sufficient nucleophile under certain conditions, especially in reactions where a highly reactive electrophile is present or where the reaction is catalyzed to enhance its rate.
Q: What factors make water a weak nucleophile?
A: Its neutral charge, high electronegativity of oxygen, and its protic nature (leading to self-solvation) all contribute to its relatively weak nucleophilicity.
Q: How can I improve water's nucleophilicity?
A: The most effective way to enhance water's nucleophilic capabilities is by increasing the concentration of hydroxide ions (OH⁻) through the addition of a base. Using aprotic solvents can also improve its reactivity.
Q: What are some examples of reactions where water acts as a nucleophile?
A: Hydrolysis of esters, amides, and other functional groups are excellent examples. Water also plays a crucial role as a nucleophile in many enzymatic reactions within biological systems.
Conclusion
Water's nucleophilicity is a complex topic. While not a potent nucleophile compared to its negatively charged counterparts, it plays a significant role in numerous chemical reactions, particularly hydrolysis and biological processes. Understanding the factors influencing its nucleophilicity—charge, electronegativity, steric hindrance, and solvent effects—is crucial for predicting its reactivity in different chemical environments. Its availability, amphoteric nature, and participation in essential reactions ensure its continued importance in chemistry and related fields. While it may not always be the best nucleophile, its ubiquitous presence and versatility solidify its role in a vast array of chemical transformations.
Latest Posts
Latest Posts
-
How To Calculate Percentage Concentration
Sep 06, 2025
-
Parametric Equations And Projectile Motion
Sep 06, 2025
-
Sample Compare And Contrast Paragraph
Sep 06, 2025
-
Controlling The Growth Of Microorganisms
Sep 06, 2025
-
Three Stages Of Signal Transduction
Sep 06, 2025
Related Post
Thank you for visiting our website which covers about Is H2o A Good Nucleophile . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.