Lithium Molar Mass G Mol
metako
Sep 12, 2025 · 6 min read
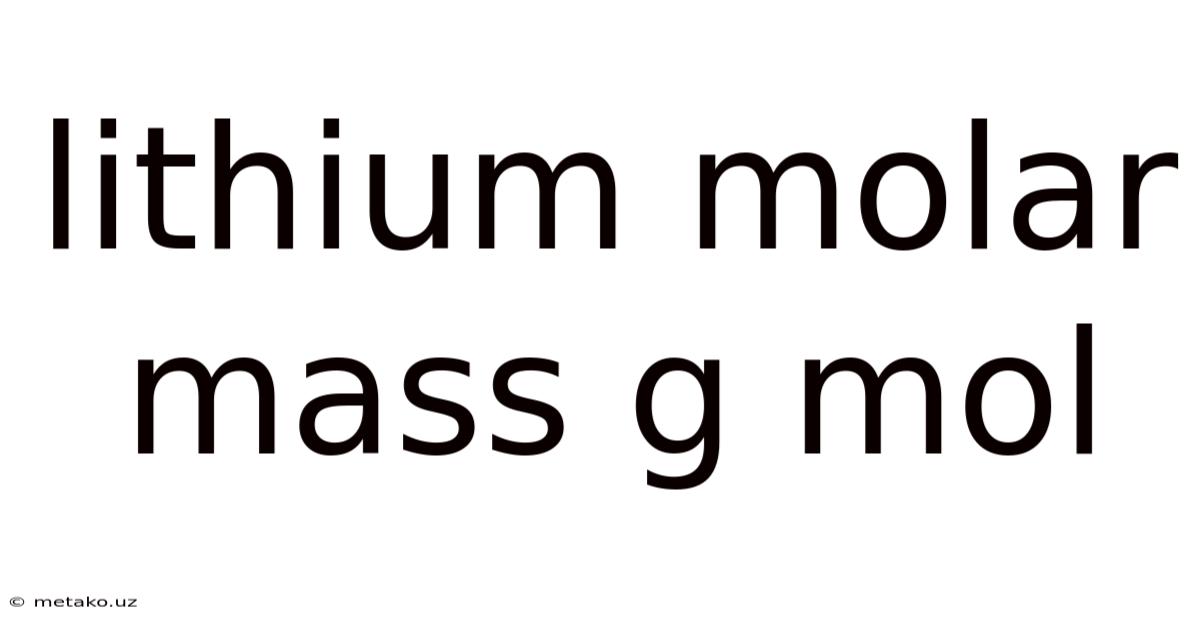
Table of Contents
Understanding Lithium's Molar Mass: A Deep Dive into Atomic Weight and its Applications
Lithium, the lightest metal, holds a significant place in various scientific fields and industrial applications. Understanding its properties, especially its molar mass (g/mol), is crucial for accurate calculations and efficient utilization. This comprehensive article will explore the concept of molar mass, delve into the specifics of lithium's atomic weight, and discuss its practical implications in different domains. We'll also address common questions and misconceptions surrounding this fundamental chemical concept.
What is Molar Mass?
The molar mass of an element or compound represents the mass of one mole of that substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This number, known as Avogadro's number, is approximately 6.022 x 10²³.
Therefore, the molar mass essentially tells us the mass (in grams) of 6.022 x 10²³ particles of a given substance. It's expressed in grams per mole (g/mol). For elements, the molar mass is numerically equal to the atomic weight (or standard atomic weight) found on the periodic table.
Determining Lithium's Molar Mass
Lithium (Li), with atomic number 3, is located in Group 1 (alkali metals) of the periodic table. Its standard atomic weight, as determined by the International Union of Pure and Applied Chemistry (IUPAC), is approximately 6.941 g/mol. This value isn't a whole number because lithium exists in nature as a mixture of two stable isotopes: lithium-6 (⁶Li) and lithium-7 (⁷Li).
-
Lithium-6 (⁶Li): This isotope constitutes about 7.6% of naturally occurring lithium and has a mass of approximately 6 atomic mass units (amu).
-
Lithium-7 (⁷Li): This isotope makes up roughly 92.4% of naturally occurring lithium and has a mass of approximately 7 amu.
The standard atomic weight of 6.941 g/mol is a weighted average of the masses of these isotopes, reflecting their natural abundance. The calculation is as follows:
(0.076 x 6 amu) + (0.924 x 7 amu) ≈ 6.94 amu
Since 1 amu is approximately equal to 1 g/mol, the molar mass of lithium is approximately 6.941 g/mol. The slight variations in reported values stem from the continuous refinement of isotopic abundance measurements.
The Importance of Accurate Molar Mass in Calculations
The accurate molar mass of lithium is essential for various stoichiometric calculations in chemistry. Here are some examples:
-
Mole-to-Mass Conversions: If you know the number of moles of lithium, you can calculate its mass using the formula:
Mass (g) = Moles x Molar Mass (g/mol)
-
Mass-to-Mole Conversions: Conversely, if you know the mass of lithium, you can determine the number of moles:
Moles = Mass (g) / Molar Mass (g/mol)
-
Chemical Reactions: In balanced chemical equations, the molar mass is crucial for determining the amounts of reactants and products involved. For instance, if you are analyzing a reaction involving lithium and another element, knowing lithium's molar mass allows you to calculate the precise amount of lithium needed to react completely with a given amount of the other element.
-
Concentration Calculations: In solutions, the molar mass is essential for calculating molarity (moles of solute per liter of solution), a crucial parameter in many chemical and biochemical experiments.
Applications of Lithium and its Molar Mass Significance
Lithium's unique properties, stemming from its low atomic weight and electronic configuration, contribute to its wide-ranging applications:
-
Batteries: Lithium-ion batteries are ubiquitous in portable electronic devices, electric vehicles, and energy storage systems. Precise knowledge of lithium's molar mass is crucial in designing these batteries, optimizing their capacity, and predicting their lifespan. The molar mass helps determine the amount of lithium required to achieve a specific energy density.
-
Ceramics and Glass: Lithium compounds are added to ceramics and glass to improve their strength, durability, and thermal properties. The molar mass is important for controlling the stoichiometry of these materials and ensuring desired characteristics.
-
Lubricants: Lithium-based greases are excellent lubricants due to their high temperature stability and water resistance. Accurate molar mass determination is crucial for producing these greases with consistent quality and performance.
-
Medicine: Lithium carbonate is used as a mood stabilizer in the treatment of bipolar disorder. The precise dosage of lithium depends on the patient's body mass and other factors, highlighting the importance of accurate molar mass calculations in pharmaceutical applications.
-
Nuclear Applications: Lithium isotopes have different nuclear properties, making them relevant in nuclear reactions. Lithium-6 is used in thermonuclear weapons, while lithium-7 is used as a coolant in nuclear reactors. The accurate molar mass is critical in these contexts for calculating reaction yields and managing nuclear processes safely and efficiently.
Frequently Asked Questions (FAQ)
Q: Is the molar mass of lithium always exactly 6.941 g/mol?
A: No, the value of 6.941 g/mol is the standard atomic weight, a weighted average reflecting the natural isotopic abundance of lithium. The precise molar mass might slightly vary depending on the specific source of lithium and its isotopic composition. However, for most practical purposes, 6.941 g/mol provides sufficient accuracy.
Q: How does the molar mass of lithium compare to other alkali metals?
A: Lithium has the lowest molar mass among the alkali metals (Li: 6.941 g/mol, Na: 22.99 g/mol, K: 39.10 g/mol, Rb: 85.47 g/mol, Cs: 132.91 g/mol). This low molar mass contributes to its unique properties, such as its high reactivity and low density.
Q: Can the molar mass of lithium change?
A: The molar mass of naturally occurring lithium remains relatively constant. However, in specific experiments involving isotopic enrichment or separation, the isotopic composition, and consequently the average molar mass, might be altered.
Conclusion
The molar mass of lithium, approximately 6.941 g/mol, is a fundamental property that plays a critical role in numerous scientific and industrial applications. Understanding its significance extends beyond simple calculations; it's crucial for designing efficient batteries, creating advanced materials, and controlling the precise dosage of lithium-based medications. This deep dive into lithium's atomic weight emphasizes its importance in various fields, highlighting the interconnectedness of fundamental chemical concepts and their real-world implications. Precise knowledge and understanding of molar mass are indispensable for advancements in many technological and medical sectors relying on lithium's unique properties. Further research into isotopic ratios and the precise determination of lithium's molar mass continues to contribute to advancements in these areas. The applications of lithium and the importance of accurately understanding its molar mass are constantly expanding as science and technology advance.
Latest Posts
Latest Posts
-
Blood Agar Of Staphylococcus Aureus
Sep 12, 2025
-
Early Selection Models Of Attention
Sep 12, 2025
-
Anatomy Of A Crayfish External
Sep 12, 2025
-
3rd Period Of Periodic Table
Sep 12, 2025
-
What Is The Anthropological Perspective
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Lithium Molar Mass G Mol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.