Noble Gas Configuration Of Gold
metako
Sep 18, 2025 · 6 min read
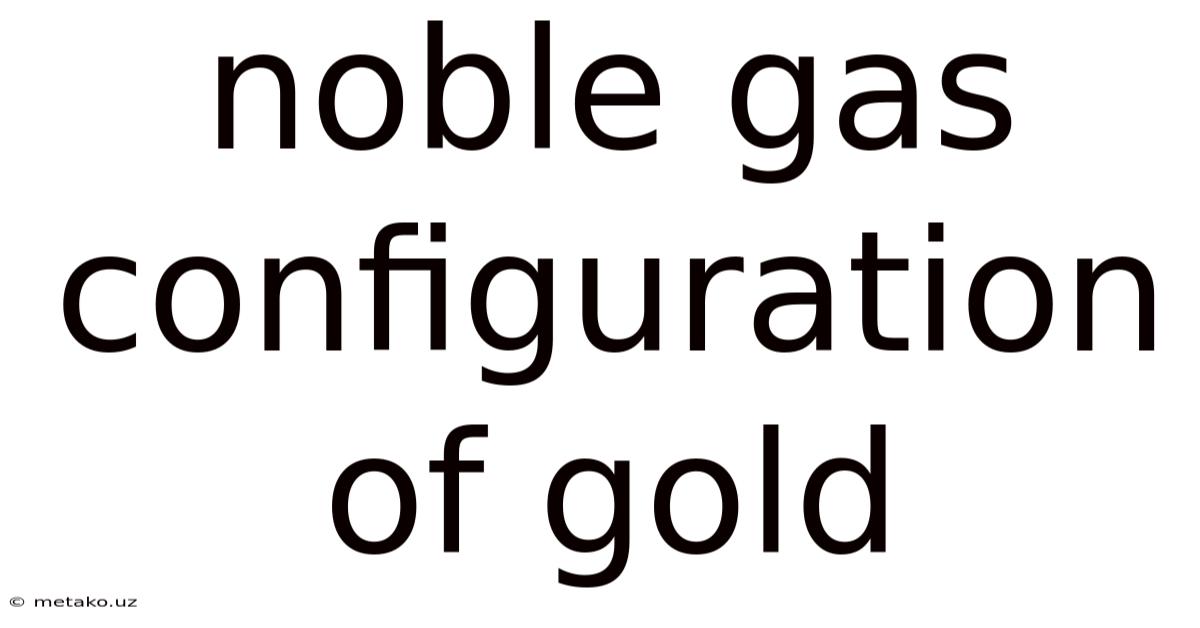
Table of Contents
Unraveling the Mystery: The Noble Gas Configuration of Gold and its Implications
Gold, a lustrous and precious metal known for its malleability and resistance to corrosion, holds a unique position in the periodic table. Its properties, so prized by humans for millennia, are intricately linked to its electronic structure, specifically its noble gas configuration. Understanding this configuration is crucial to comprehending gold's chemical behavior, its catalytic activity, and its distinctive physical characteristics. This article delves deep into the noble gas configuration of gold, exploring its intricacies and implications. We'll unravel the complexities behind its seemingly simple electron arrangement, examining the relativistic effects and its impact on gold's unique properties.
Understanding Electron Configurations: A Quick Refresher
Before diving into the specifics of gold's configuration, let's revisit the basics. The electron configuration of an atom describes how electrons are distributed among its various energy levels and sublevels. These configurations follow specific rules, dictated by quantum mechanics, including the Aufbau principle (filling orbitals from lowest to highest energy), Hund's rule (maximizing unpaired electrons in a subshell), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
Elements are organized in the periodic table based on their electron configurations. Each period represents a principal energy level, while groups reflect similar valence electron configurations, hence similar chemical behavior. Noble gases, found in group 18, possess a stable configuration with filled valence shells, making them exceptionally unreactive. This stable configuration is often denoted as a noble gas core.
Gold's Electron Configuration: A Closer Look
Gold (Au), with an atomic number of 79, has 79 electrons. Its complete electron configuration is typically written as:
[Xe] 4f¹⁴ 5d¹⁰ 6s¹
This means that the inner electrons of gold mimic the configuration of Xenon (Xe), a noble gas. This [Xe] core represents 54 electrons. The remaining 25 electrons occupy the 4f, 5d, and 6s orbitals. The outermost electrons, residing in the 6s and 5d orbitals, are the valence electrons, which determine gold's chemical reactivity. Notice that the 6s orbital only has one electron, while the 5d orbital is completely filled. This seemingly anomalous configuration is crucial in understanding gold's unique behavior.
The Role of Relativistic Effects
While the standard Aufbau principle predicts the 6s orbital filling before the 5d, gold's electron configuration deviates slightly due to relativistic effects. These effects become increasingly important for heavier elements like gold. The inner electrons, moving at significant speeds, experience a considerable increase in mass due to Einstein's theory of special relativity. This increase in mass leads to:
- Orbital Contraction: The s and p orbitals, which are closer to the nucleus, experience a greater contraction due to the increased mass of the inner electrons.
- Orbital Expansion: The d and f orbitals, being further from the nucleus, experience a slight expansion.
These relativistic effects have a profound impact on the energy levels of the 6s and 5d orbitals. The 6s orbital contracts significantly, lowering its energy and making it more stable. The 5d orbital expands slightly, increasing its energy. This energy shift results in the 6s orbital being filled with only one electron, while the 5d orbital becomes completely filled, leading to the observed [Xe] 4f¹⁴ 5d¹⁰ 6s¹ configuration.
Implications of Gold's Noble Gas Configuration
Gold's unique noble gas configuration, influenced by relativistic effects, dictates several of its important properties:
-
Relatively Low Reactivity: While not as inert as noble gases, gold's filled 5d subshell provides significant stability. This makes it less reactive compared to other transition metals. It resists oxidation and corrosion, a key factor contributing to its value as a precious metal. Its relatively low reactivity also explains its occurrence in its native form in nature.
-
Gold's Color: The interaction of light with the 5d and 6s electrons, influenced by relativistic effects, leads to gold's characteristic yellow color. This is unusual for transition metals, which often exhibit various colors, including vibrant blues and greens. The relativistic contraction of the 6s orbitals modifies the electron transitions, impacting the absorption and reflection of light, resulting in the unique yellow hue.
-
Catalytic Activity: Despite its relative inertness, gold exhibits surprising catalytic activity, particularly in nanoparticle form. This is attributed to the relatively easily accessible 6s electron, which can participate in catalytic reactions. This makes gold nanoparticles extremely effective catalysts in various chemical processes, including oxidation and reduction reactions.
-
Malleability and Ductility: Gold's ability to be easily hammered into thin sheets (malleability) and drawn into wires (ductility) is also linked to its electron configuration and the way the electrons are delocalized throughout the metallic lattice. This delocalization allows the atoms to slide past each other without disrupting the metallic bonding.
Gold's Oxidation States: A Deeper Dive
Despite its relatively low reactivity, gold can exhibit several oxidation states. While the +1 (aurous) and +3 (auric) oxidation states are the most common, other less stable states, such as +2 and +5, have also been observed in specific compounds. The stability of these oxidation states is influenced by the relativistic effects on the 6s and 5d orbitals. The energy difference between these orbitals determines the ease with which gold loses electrons to form different oxidation states.
Frequently Asked Questions (FAQ)
Q1: Why is gold's electron configuration unusual?
A1: Gold's electron configuration is unusual because of relativistic effects. These effects alter the energy levels of the 6s and 5d orbitals, leading to the unexpected filling of one electron in the 6s orbital while the 5d orbital remains filled. This deviation from the standard Aufbau principle is unique to heavier elements.
Q2: How does the noble gas configuration impact gold's reactivity?
A2: The filled 5d subshell in gold contributes to its relatively low reactivity. However, the single electron in the 6s orbital can participate in chemical reactions, leading to the formation of various compounds, particularly when gold is in the +1 oxidation state.
Q3: What role do relativistic effects play in gold's color?
A3: Relativistic effects significantly influence gold's color. The relativistic contraction of the 6s orbital modifies the electron transitions, affecting the absorption and reflection of light and resulting in its characteristic yellow color.
Q4: Why is gold so malleable and ductile?
A4: The delocalized nature of the electrons in the gold metallic lattice, influenced by its electronic configuration, allows the atoms to slide past each other easily without disrupting the metallic bonding. This contributes to its remarkable malleability and ductility.
Conclusion: A Unique Metal with a Unique Configuration
Gold's noble gas configuration, a fascinating interplay of standard electronic structure rules and relativistic effects, is fundamental to understanding its unique properties. The seemingly simple [Xe] 4f¹⁴ 5d¹⁰ 6s¹ configuration is far from simple, revealing a complex interplay of forces within the atom. This configuration, shaped by relativistic effects, accounts for gold’s low reactivity, distinctive color, catalytic properties, and exceptional malleability and ductility. Its study underscores the importance of considering relativistic effects when examining the properties of heavier elements and highlights the intricate relationship between atomic structure and macroscopic behavior. The journey into understanding gold's electronic structure is not just an academic exercise; it offers critical insights into the behavior of this valuable and fascinating element. Further research continues to unveil the subtle nuances of relativistic effects and their influence on gold's captivating chemistry and physics.
Latest Posts
Latest Posts
-
What Color Is Cell Membrane
Sep 18, 2025
-
Dot Product In Spherical Coordinates
Sep 18, 2025
-
Polar To Cartesian Equation Converter
Sep 18, 2025
-
Reinforcement Cell Structures Answer Key
Sep 18, 2025
-
U Sub With Definite Integrals
Sep 18, 2025
Related Post
Thank you for visiting our website which covers about Noble Gas Configuration Of Gold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.