Noble Gas Configuration Of Iodine
metako
Sep 16, 2025 · 6 min read
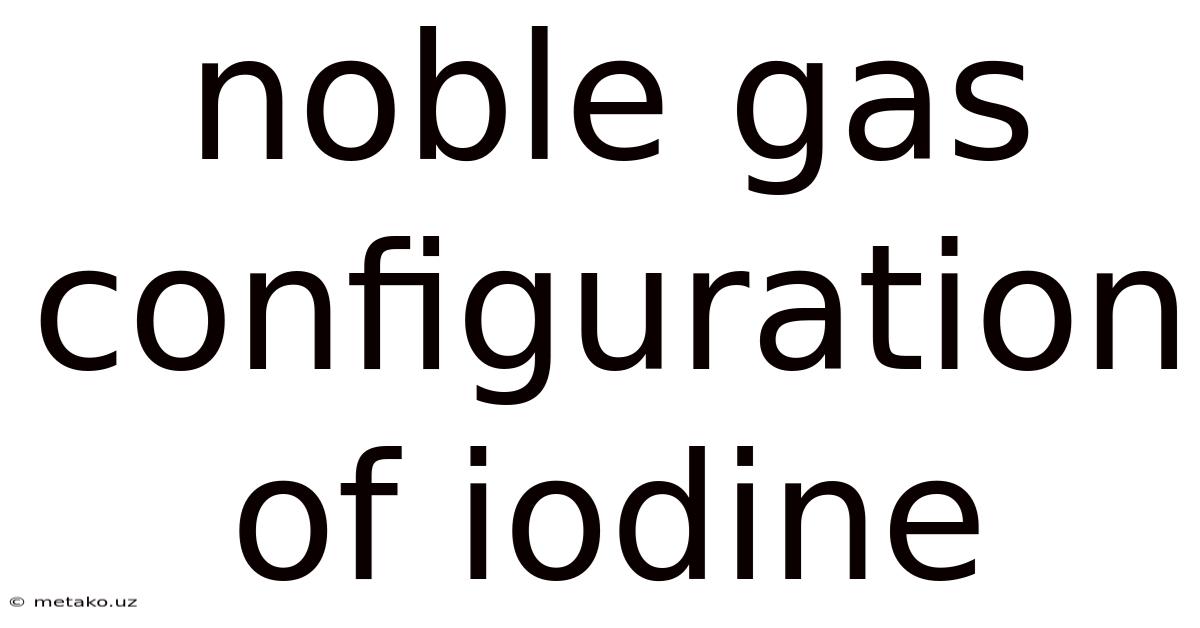
Table of Contents
Understanding the Noble Gas Configuration of Iodine
Iodine, a fascinating element crucial for human health and various industrial applications, boasts an electron configuration that significantly impacts its chemical properties. Understanding its noble gas configuration is key to grasping its reactivity and behavior. This article will delve deep into iodine's electronic structure, explaining its noble gas configuration, its implications for chemical bonding, and exploring related concepts in a comprehensive and accessible manner. We will also address frequently asked questions to ensure a thorough understanding of this important topic.
Introduction to Iodine and Electronic Configuration
Iodine (I), a halogen element with atomic number 53, is a lustrous, purplish-black solid at room temperature. Its position in the periodic table, in Group 17 (also known as the halogens), and Period 5, dictates its electronic structure and consequently, its chemical properties. The electronic configuration represents the arrangement of electrons within the atom's various energy levels and sublevels. Knowing this configuration is fundamental to understanding its chemical behavior, particularly its tendency to form chemical bonds.
Determining Iodine's Electron Configuration
The electron configuration of an atom describes how its electrons are distributed among its orbitals. We can predict the electron configuration of iodine using the Aufbau principle, Hund's rule, and the Pauli exclusion principle. These rules dictate how electrons fill atomic orbitals in order of increasing energy, with each orbital holding a maximum of two electrons with opposite spins.
Following these principles, iodine's full electron configuration is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁵. This notation indicates the number of electrons in each subshell. For instance, '1s²' means two electrons occupy the 1s orbital, '2s²' means two electrons are in the 2s orbital, and so on.
The Noble Gas Configuration of Iodine
Instead of writing out the full electron configuration every time, we can use a shorthand notation that simplifies the representation. This is where the noble gas configuration comes in. Noble gases, located in Group 18 of the periodic table, are characterized by their exceptionally stable electron configurations (full valence shells). We can represent the inner core electrons of iodine using the noble gas that precedes it in the periodic table – Krypton (Kr). Krypton has an electron configuration of 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶.
Therefore, iodine's noble gas configuration is written as [Kr]5s²4d¹⁰5p⁵. This notation signifies that iodine has the same electron configuration as krypton for its inner electrons, plus additional electrons in the 5s, 4d, and 5p subshells. This simplified notation highlights the valence electrons, which are the electrons involved in chemical bonding. In iodine's case, the valence electrons are those in the 5s and 5p subshells (a total of 7 electrons).
Implications of Iodine's Noble Gas Configuration and Valence Electrons
The noble gas configuration and, more importantly, the presence of seven valence electrons are crucial in determining iodine's chemical behavior. Elements strive to achieve a stable electron configuration, ideally resembling that of a noble gas (a full octet, or eight valence electrons). Since iodine has seven valence electrons, it is one electron short of having a full octet. This drives its strong tendency to gain an electron, forming a stable iodide ion (I⁻) with a full octet, hence its high electronegativity.
This inclination to gain an electron explains iodine's reactivity. It readily forms ionic bonds with electropositive metals, readily accepting an electron to complete its octet. For example, in sodium iodide (NaI), sodium (Na) donates an electron to iodine (I), resulting in the formation of Na⁺ and I⁻ ions, held together by electrostatic attraction.
Iodine can also form covalent bonds, sharing electrons with other nonmetals to achieve a stable octet. In molecules like I₂, two iodine atoms share one electron pair, forming a single covalent bond.
Further Exploring Iodine's Chemical Properties: Beyond the Noble Gas Configuration
While the noble gas configuration provides a foundational understanding of iodine's reactivity, it's crucial to acknowledge other factors that influence its chemical behavior. These include:
- Atomic size: Iodine is a relatively large atom, leading to a weaker attraction between its nucleus and valence electrons. This makes it less electronegative than other halogens like fluorine and chlorine.
- Electron shielding: The presence of inner electrons shields the valence electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by the valence electrons. This also contributes to iodine's larger atomic size and lower electronegativity.
- Ionization energy: The energy required to remove an electron from an iodine atom is relatively low compared to other halogens. This is consistent with the weaker attraction between the nucleus and valence electrons due to increased atomic size and shielding.
Iodine's Role in Biology and Industry
Iodine's unique properties make it essential in various biological and industrial applications. In biology, iodine is crucial for the production of thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These hormones regulate metabolism and are essential for normal growth and development. Iodine deficiency can lead to goiter and hypothyroidism.
Industrially, iodine finds applications in various areas, including:
- Disinfection: Iodine compounds are used as disinfectants due to their antimicrobial properties.
- Photography: Silver iodide (AgI) is used in photographic film.
- Catalysis: Iodine acts as a catalyst in some chemical reactions.
- Organic synthesis: Iodine and its compounds play important roles in the synthesis of various organic molecules.
Frequently Asked Questions (FAQ)
Q1: What is the difference between electron configuration and noble gas configuration?
A1: The electron configuration shows the complete distribution of electrons in all energy levels and sublevels of an atom. The noble gas configuration is a simplified version, using the symbol of the preceding noble gas to represent the inner core electrons, followed by the configuration of the remaining valence electrons.
Q2: Why is the noble gas configuration important?
A2: The noble gas configuration highlights the valence electrons – the electrons involved in chemical bonding. It simplifies the representation of an atom's electronic structure and allows for easier prediction of its chemical behavior.
Q3: Can iodine form more than one ion?
A3: While the iodide ion (I⁻) is the most common ion formed by iodine, it can also form other ions under specific conditions. However, these are less stable and less common than the iodide ion.
Q4: How does the size of iodine affect its reactivity?
A4: Iodine's larger atomic size compared to other halogens results in weaker attraction between the nucleus and valence electrons, making it less electronegative and more reactive than expected, relative to the other halogens. This also explains its lower ionization energy.
Q5: What are some common compounds of iodine?
A5: Some common compounds of iodine include sodium iodide (NaI), potassium iodide (KI), iodine monochloride (ICl), and hydrogen iodide (HI).
Conclusion
Iodine's noble gas configuration, [Kr]5s²4d¹⁰5p⁵, provides a fundamental understanding of its chemical behavior. Its seven valence electrons drive its tendency to gain an electron, forming the stable iodide ion (I⁻). Understanding this configuration, along with other factors like atomic size and electronegativity, is crucial to appreciating iodine's diverse applications in biology and industry. This knowledge underscores the importance of mastering electron configurations for a deeper understanding of chemical reactivity and the properties of elements. Further exploration of iodine's chemistry will reveal its multifaceted role in various scientific fields.
Latest Posts
Latest Posts
-
How Do You Graph Y
Sep 16, 2025
-
Exact Equation Differential Equations Examples
Sep 16, 2025
-
Reaction Of Naoh With Hcl
Sep 16, 2025
-
Cite The Law Of Reflection
Sep 16, 2025
-
Lcm Of 16 And 20
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about Noble Gas Configuration Of Iodine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.