Vmax Equals Kcat Times E
metako
Sep 22, 2025 · 6 min read
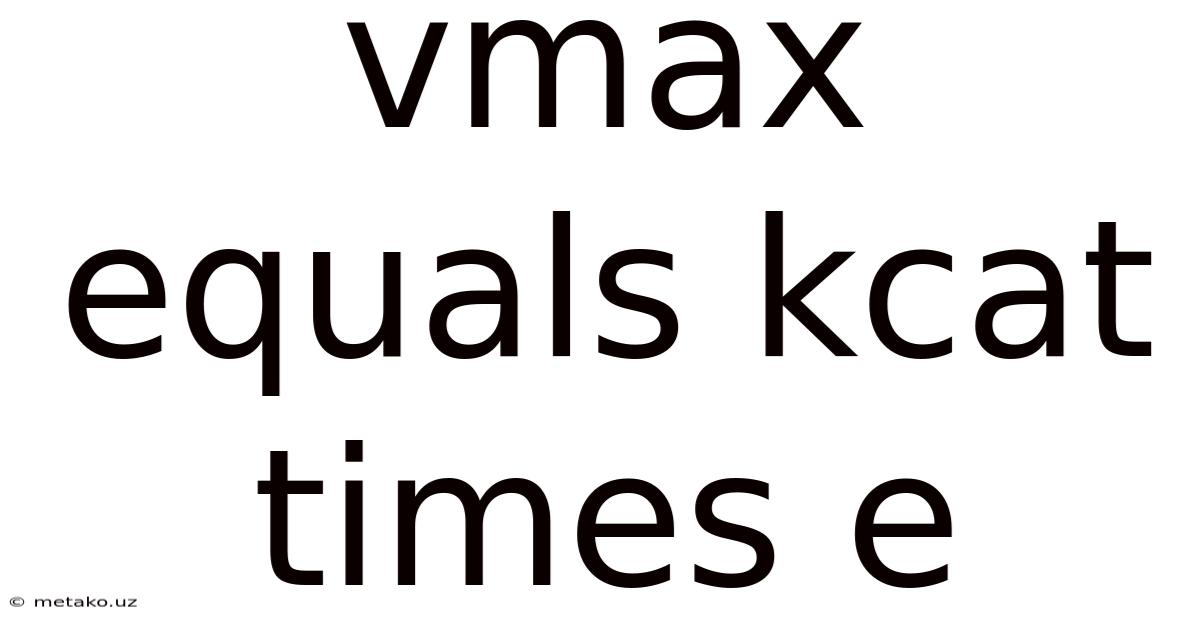
Table of Contents
Understanding Vmax: The Enzyme's Maximum Velocity and the Michaelis-Menten Equation
The equation Vmax = kcat[E] is a cornerstone of enzyme kinetics, a crucial area of biochemistry. It elegantly connects the maximum velocity (Vmax) of an enzyme-catalyzed reaction to the enzyme's turnover number (kcat) and the total enzyme concentration ([E]). Understanding this equation is fundamental to comprehending how enzymes function and how their activity can be modulated. This article will delve deep into this equation, exploring its components, its derivation from the Michaelis-Menten equation, its significance in understanding enzyme behavior, and addressing common misconceptions.
Introduction to Enzyme Kinetics and the Michaelis-Menten Equation
Enzymes are biological catalysts that significantly accelerate the rate of biochemical reactions. Enzyme kinetics studies the rates of these reactions and the factors that influence them. A central concept in enzyme kinetics is the Michaelis-Menten equation, which describes the relationship between the initial reaction velocity (V₀) and the substrate concentration ([S]). The equation is:
V₀ = (Vmax[S]) / (Km + [S])
where:
- V₀: Initial reaction velocity (rate of product formation at the beginning of the reaction).
- Vmax: Maximum reaction velocity – the theoretical maximum rate achieved when the enzyme is saturated with substrate.
- [S]: Substrate concentration.
- Km: Michaelis constant – represents the substrate concentration at which the reaction velocity is half of Vmax. It's an indicator of the enzyme's affinity for its substrate; a lower Km indicates higher affinity.
Deriving Vmax = kcat[E] from the Michaelis-Menten Equation
While the Michaelis-Menten equation provides a valuable framework, it doesn't directly explain what determines Vmax. To understand this, we need to introduce the concept of turnover number (kcat), also known as the catalytic constant.
kcat represents the number of substrate molecules converted to product per enzyme molecule per unit time when the enzyme is saturated with substrate. In simpler terms, it's a measure of the enzyme's catalytic efficiency. A higher kcat indicates a faster enzyme.
Under saturating substrate conditions ([S] >> Km), the Michaelis-Menten equation simplifies to:
V₀ ≈ Vmax
This is because the denominator (Km + [S]) is essentially equal to [S] when [S] is much larger than Km. Therefore, the equation becomes:
Vmax ≈ Vmax[S] / [S] = Vmax
Now, let's consider the enzymatic reaction mechanism. When the enzyme is saturated with substrate, every enzyme molecule is actively converting substrate to product. The rate of product formation under these conditions is directly proportional to the enzyme concentration and the turnover number:
Vmax = kcat[E]
This equation tells us that the maximum velocity of an enzyme-catalyzed reaction is determined by two factors:
- kcat (Turnover number): This intrinsic property of the enzyme reflects its catalytic efficiency. A higher kcat means the enzyme can process more substrate molecules per unit time.
- [E] (Enzyme concentration): A higher enzyme concentration means more enzyme molecules are available to catalyze the reaction, leading to a higher Vmax.
Understanding the Components of the Equation: kcat and [E]
Let's delve deeper into the meaning and significance of kcat and [E]:
kcat (Turnover Number):
- Definition: The number of substrate molecules converted to product per enzyme molecule per unit time when the enzyme is saturated with substrate.
- Units: s⁻¹ (per second)
- Significance: It's a measure of the enzyme's catalytic efficiency. A higher kcat indicates a faster enzyme, implying a more efficient catalytic mechanism.
- Determination: kcat can be determined experimentally by measuring Vmax and the total enzyme concentration ([E]). kcat = Vmax/[E].
[E] (Enzyme Concentration):
- Definition: The total concentration of the enzyme in the reaction mixture.
- Units: M (molar) or µM (micromolar)
- Significance: A higher enzyme concentration directly increases the number of enzyme molecules available to bind substrate and catalyze the reaction. This directly influences Vmax.
- Determination: The enzyme concentration can be determined through various biochemical methods, such as Bradford assay, BCA assay, or UV-Vis spectroscopy.
The Significance of Vmax = kcat[E]
The equation Vmax = kcat[E] provides crucial insights into enzyme behavior and allows for several applications:
- Enzyme characterization: Determining kcat and Vmax is essential for characterizing an enzyme and comparing its catalytic efficiency to other enzymes.
- Drug design: Understanding enzyme kinetics is vital in drug development. Inhibitors designed to target enzymes often aim to reduce Vmax or increase Km.
- Metabolic engineering: Manipulating enzyme expression levels ([E]) or engineering enzymes with improved kcat can be used to optimize metabolic pathways in biotechnology.
- Understanding enzyme regulation: Factors that affect enzyme concentration or activity can be studied through their impact on Vmax.
Applications and Examples
The equation finds wide application in various areas:
- Pharmacology: The efficacy of many drugs depends on their ability to inhibit specific enzymes. Measuring the impact of a drug on Vmax helps determine its potency and mechanism of action. A competitive inhibitor might not affect kcat but will increase Km, while a non-competitive inhibitor might decrease Vmax but not affect Km.
- Biotechnology: In industrial enzyme applications, optimizing enzyme production and improving catalytic efficiency (kcat) are crucial factors in maximizing yield and reducing costs.
- Clinical diagnostics: Measuring enzyme activity in blood or tissue samples is a common diagnostic tool for various diseases. Changes in Vmax can indicate organ damage or disease progression.
Frequently Asked Questions (FAQ)
Q1: What happens if the enzyme concentration is doubled?
A1: Doubling the enzyme concentration ([E]) will double the Vmax, provided that the substrate is in excess and the enzyme is not already saturated. This is because there are twice as many enzyme molecules available to catalyze the reaction.
Q2: What happens if kcat is doubled?
A2: Doubling kcat would also double Vmax, assuming the enzyme concentration remains constant. This indicates an improvement in the enzyme's catalytic efficiency. Each enzyme molecule now works twice as fast.
Q3: What if [S] is not much greater than Km?
A3: If [S] is not significantly larger than Km, the simplified Michaelis-Menten equation (V₀ ≈ Vmax) doesn't hold true. The full Michaelis-Menten equation must be used to calculate the initial reaction velocity. Vmax is still defined by kcat[E], but the reaction will not be operating at its maximum velocity.
Q4: How is kcat related to Km?
A4: While both kcat and Km are important kinetic parameters, they provide different information about the enzyme. kcat reflects the enzyme's turnover rate at saturation, while Km reflects the enzyme's affinity for its substrate. The ratio kcat/Km is often used as a measure of catalytic efficiency, considering both turnover rate and substrate affinity. A higher kcat/Km value indicates a more efficient enzyme.
Q5: Can Vmax be infinite?
A5: No. Vmax represents the maximum achievable rate of the reaction under specific conditions. While it can be very high, it’s always finite because the reaction is limited by the number of enzyme molecules and their inherent catalytic rate (kcat).
Conclusion
The equation Vmax = kcat[E] is a powerful tool for understanding enzyme kinetics and catalysis. It provides a direct link between the maximum velocity of an enzyme-catalyzed reaction, the enzyme's intrinsic catalytic efficiency (kcat), and the enzyme concentration ([E]). This equation is fundamental to comprehending how enzymes function, how their activity can be regulated, and how they can be engineered for biotechnological applications. Mastering this equation and its components is crucial for anyone working in biochemistry, molecular biology, or related fields. Further exploration into enzyme inhibition kinetics, allosteric regulation, and multi-substrate enzyme reactions will build upon this foundational understanding.
Latest Posts
Latest Posts
-
Characters In Uncle Toms Cabin
Sep 22, 2025
-
What Makes A Strong Acid
Sep 22, 2025
-
Strongest To Weakest Intermolecular Forces
Sep 22, 2025
-
Deviations From Ideal Gas Law
Sep 22, 2025
-
Bronsted Lowry Acid Base Pair
Sep 22, 2025
Related Post
Thank you for visiting our website which covers about Vmax Equals Kcat Times E . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.